This site is intended for UK Healthcare Professionals only.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellowcard in the Google Play or Apple App Store. Adverse events should also be reported to Cipla (EU) Ltd on 0800 0472144, drugsafety@cipla.com
It’s not just the dose counter that counts with Bibecfo® from Cipla1-4

Bibecfo indications1

Bibecfo 100/6mcg and 200/6mcg are indicated in the regular treatment of asthma where use of a combination product (inhaled corticosteroid and long-acting β₂-agonist) is appropriate:
- patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled rapid-acting β₂-agonist
or
- patients already adequately controlled on both inhaled corticosteroids and long-acting β₂-agonists

Bibecfo 100/6mcg is indicated in the symptomatic treatment of patients with severe COPD (FEV1 <50% predicted normal) and a history of repeated exacerbations, who have significant symptoms despite regular therapy with long-acting bronchodilators.
Bibecfo 100/6mcg and 200/6mcg are indicated in adults 18 years and above. For Bibecfo 100/6mcg in asthma, no recommendation on a dose can be made in children and adolescents 17 years and under.
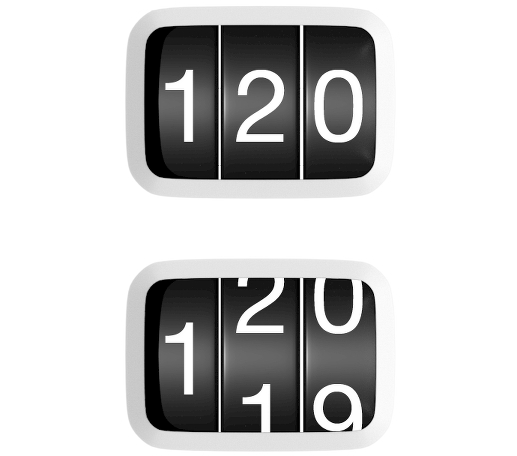
With Bibecfo, you can count on...
A pMDI with a precise dose counter1
- Like Fostair (beclometasone/formoterol), Bibecfo utilises a dose counter that counts down by one each time a puff of medicine is released, meaning patients can always be sure of exactly how many doses they have left1,5-7
A familiar inhaler technique consistent with the market originator1,5-7
- Fostair users can transition confidently to Bibecfo 100/6mcg or 200/6mcg with familiar step-by-step instructions1,5-7
The same doses, formulation and excipients as the market originator1,5-7
- Bibecfo is an extrafine beclometasone formulation available in two doses - 100/6mcg and 200/6mcg. Bibecfo has the same active ingredients (beclometasone/formaterol) and excipients (hydrochloric acid, norflurane and ethanol anhydrous) as Fostair1,5-7
Bibecfo 100/6mcg can be used as both a Maintenance and Reliever Therapy1,8
- For patients who are highly symptomatic or have severe exacerbations or uncontrolled asthma on low-dose ICS/formoterol, the latest guidelines recommend low-dose Maintenance and Reliever Therapy (MART), using a single inhaler for daily asthma control and symptom relief8
- For patients who are uncontrolled on low-dose MART, moderate-dose MART should be offered8
- Bibecfo’s dose counter tracks individual puffs, helping patients monitor usage and remaining doses – crucial for a MART regimen1,8
A sustainable, low-cost solution that will remain consistently priced in the future1,2,4
- At less than half the price of Fostair (beclometasone/formoterol), Bibecfo could make budgets go further to optimise more patients’ care1,2,5
- With end-to-end control over production – from R&D and manufacturing to supply – Cipla can support a sustainable price that will not increase in the future4
Fostair is a registered trademark of Chiesi Ltd.
Bibecfo dose regimen1
Adults 18 years and older:1
100/6mcg
Asthma (Maintenance therapy):
One or two inhalations twice daily
Maximum daily dose is 4 inhalations
Asthma (Maintenance and reliever therapy):
One inhalation twice daily with one additional inhalation as needed in response to symptoms. If symptoms persist after a few minutes, an additional inhalation should be taken
Maximum daily dose is 8 inhalations
COPD:
Two inhalations twice daily
200/6mcg
Asthma (Maintenance therapy):
Two inhalations twice daily
Maximum daily dose is 4 inhalations
Side effect profile of Bibecfo1
Patients using Bibecfo may experience side effects.
As Bibecfo contains beclometasone dipropionate and formoterol, the type and severity of adverse reactions associated with each of the compounds may be expected. There is no incidence of additional adverse events following concurrent administration of the two compounds.
As with other inhalation therapy, paradoxical bronchospasm may occur.
Common adverse events associated with beclometasone dipropionate and formoterol - either individually or as combination therapy - are listed below.
| System Organ Class | Adverse Reaction | Frequency |
|---|---|---|
| Infections and Infestations | Pharyngitis, oral candidiasis, pneumonia* (in COPD patients) | Common |
| Nervous system disorders | Headache | Common |
| Respiratory, thoracic and mediastinal disorders | Dysphonia | Common |
*One related non-serious case of pneumonia was reported by one patient treated with beclometasone dipropionate/formoterol in a pivotal clinical trial in COPD patients.
Frequencies are defined as: common (≥1/100 to <1/10)
For more information about side effects, please refer to the Summary of Product Characteristics.
COPD = chronic obstructive pulmonary disease; FEV1 = forced expiratory volume; ICS = inhaled corticosteroid; MART = maintenance and reliever therapy; pMDI = pressurised metered dose inhaler; R&D = research and development

